Overview
Cancer is one of the world’s largest causes of health problems. The overall number of deaths caused by cancer worldwide is 10.8 million. Out of which the deaths caused by Breast cancer is a total of 7,00,660. Breast cancer is the most common cancer worldwide in women, where every 4 out of 1 case consists of breast cancer. The estimated new cases of women who have Breast Cancer in 2022 are 2,87,850 which is 15% of all new cancer cases. And the estimated number of deaths in 2022 is 43,240, which forms 7.1% of all cancer deaths.
Studies prove that 99% of women who receive the treatment at their early stages live for five years longer after being diagnosed.
FDA approved their first targeted therapy for patients with HER2 low breast cancer in August 2022, which cannot be surgically removed. The new FDA-approved drug for breast cancer treatment is Trastuzumab Deruxtecan (T-DXd).
What are you thinking? If we already have breast cancer treatment then what is the need for another treatment for breast cancer?
Existing Breast Cancer Treatment & Need for New Advanced Treatment
Research states that chemotherapy treatment does not benefit breast cancer. Breast cancer treatment combines surgery, radiation, and sometimes medications that can cure cancer in its early stages. Surgery is usually the first step in treating early breast cancer.
| Treatment Options | When Required |
| Surgery | Where the cancer tissues are removed |
| Radiation Therapy | It is used to destroy any cancer cells that remain after surgery |
| Hormonal Therapy | It abstains the cancer cells from getting the hormones they need to grow. |
| Biological Therapy | Used to kill targeted cancer cells |
| Chemotherapy | Used to shrink cancer cells. |
Are you thinking about what HER 2 breast cancer is? Here is your answer ahead
What is HER2 Low Breast Cancer?
Tumors with reduced quantities of the HER2 protein on their surface are referred to as HER2-low tumors. An estimated 50% to 60% of all breast cancers are caused by these tumors. Immunohistochemistry is a laboratory procedure that checks whether HER2 is present in a tissue sample at low levels.
The overproduction of HER2 on tumor cells promotes the growth of HER2-positive breast cancer and other malignancies. The relevance of the HER2 protein in HER2-low cancers is currently unclear. Because HER2 target medications were ineffective in treating tumors with low HER2 levels, these tumors have historically been labeled as HER2-negative. Because of this, HER2-low tumors have previously received the same care as HER2-negative malignancies.
More About Trastuzumab Deruxtecan (T-DXd)
Based on a clinical trial conducted by Memorial Sloan Kettering Cancer Center, the FDA has approved the novel medication Trastuzumab Deruxtecan (T-DXd) for breast cancer treatment. Medical oncologist Dr. Shanu Modi presented her findings at the American Society of Clinical Oncology (ASCO) meeting in 2022.
Dr. Modi says. “Although this trial focused on patients with breast cancer, we believe that these results could also have implications for the future treatment of people with other types of cancer that express HER2 at low levels.”
How did the New Drug Study work?
Many patients who previously responded well to trastuzumab subsequently developed drug resistance. So in recent years, scientists have developed new drugs that target HER2 cancers more effectively. T-DXD is one of these medications, which the FDA initially approved in 2019 for treating patients with metastatic, HER2-positive breast cancer. Precisely to say who had stopped responding to existing HER2 medications. That approval was based on a study published in the NEJM.
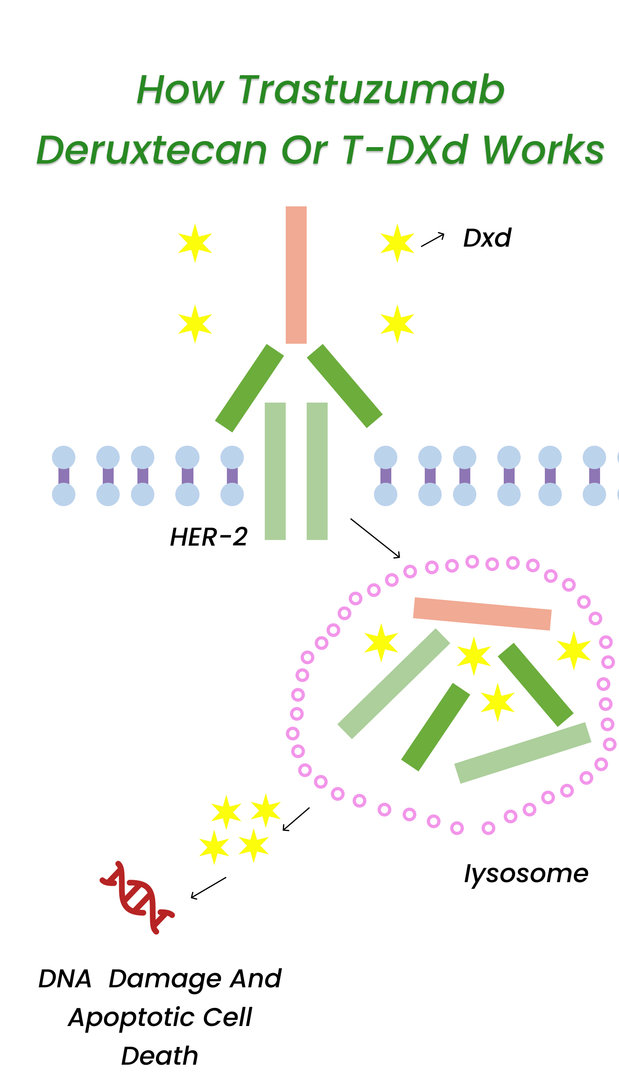
Scientists are constantly researching and developing new ways to cure breast cancer worldwide. These clinical trials helped explore new treatment options and understand what treatments suit what types of patients.
In the first trial, patients received T-DXD, targeting a protein called HER2. Trastuzumab directly blocks growth signals sent by HER2, but T-DXD works differently. This medication delivers trastuzumab to the tumor location and a payload of chemotherapy. As a result, T-DXD has much more potential than traditional trastuzumab.
The targeted drug held their cancer in check for nearly twice as long and increased their survival by about 35%.
The findings from this international, multicenter trial, DESTINY-Breast04, were also published in The New England Journal of Medicine (NEJM) on June 5, 2022.
According to Dr. Modi and her team's research, it could be effective against tumors with low (but not absent) levels of HER2. More than 60% of tumors that are conventionally classified as HER2-negative were referred to as "HER2-low" in this study.
Several patients with HER2-low, advanced-stage cancer were tested with the T-DXD medication. In phase 3 DESTINY-Breast04 study, 557 metastatic breast cancer patients enrolled who had HER2 low tumors. One third of them received conventional chemotherapy, while on the other hand, two thirds received T-DXD.
The trial showed an improvement in the population with this therapy to live longer compared to the other standard care treatments. Research showed that those who received T-DXD did significantly better than those who received standard chemotherapy. In addition, study showed that their survival rate was much better than the rest at that stage, approximately 23.9 months compared with 17.5 months for those in the chemotherapy group.
Can T-DXD treat other cancer patients?
Yes. As per the experts it can.
The subsets of stomach, lung, and colorectal cancers are known to express HER2 as well. T-DXd is also approved for patients with advanced gastric cancer and is being experimented with for other types of tumors. Metastatic is the advanced stage of breast cancer which can now be cured with this new medication.
According to the research, in the US, there is an estimate of around 1,68,000 people surviving stage 4 breast cancer. Trastuzumab Deruxtecan or T-DXd, which is recently an FDA-approved medication for breast cancer, has shown positive outcomes and is extending the lives of the existing patients in this segment.
HER2 positive, which accounts for 15% to 20%, is the status in breast cancer when this protein is present in large quantities on the cell's surface, making cancer more aggressive.
HER2-negative cancers make up a large variety of cancers, and many of them have low levels of proteins, making HER2 low.
“The results of this trial are practice-changing and redefine how a large population of patients with metastatic disease will be treated.” said by Dr. Shanu Modi

Are there any side effects associated with the new medication?
Depending on person to person, like every drug Trastuzumab Deruxtecan has certain minor side effects that have been observed. Everybody reacts in some way or another to different types of drugs, and some people can have more side effects than others. These side effects can usually be managed and the ones mentioned below may not necessarily affect everybody.
Let's check the side effects ahead
1. Lowering of Blood Counts: The number of blood cells in the body can momentarily change due to trastuzumab deruxtecan. To assess your blood count, you'll have routine blood tests.
Your subsequent cycle of therapy can be postponed, or the dose might be decreased if the number of blood cells is too low.
Doctors frequently adjust a drug's dose by lowering it to fit a patient's needs and to manage its adverse effects. This may enable some people to continue receiving treatment for longer.
2. Risk of Infection: A low count of white blood cells can increase the risk of infection.
* A temperature that is high (above 37.5°C), low (under 36°C), or whatever your treatment team has recommended
* sudden illness despite having a normal temperature
* symptoms of an infection, such as a sore throat, cough, frequent urination, or feeling chilly or queasy
3. Anemia: Not having enough red blood cells is Anaemia. You may especially experience the feeling of being exhausted, out of breath and dizzy.
4. Bruising and bleeding: Trastuzumab Deruxtecan has the ability to lower platelet counts, which aids in blood clotting. Additionally, you can bruise more readily, experience nosebleeds, or experience bleeding gums while brushing your teeth. If you have any of these symptoms, let your doctors know about them.
5. Changes in the Heart: By weakening the cardiac muscle, trastuzumab deruxtecan may result in alterations to the heart. For most people, this is transient, but for a select few, it can be lifelong.
If you experience any of the following:
- Breathlessness
- Chest pain
- Alterations in your heartbeat
- Enlarged ankles
Your treatment team will schedule a heart (cardiac) function test before you begin treatment to ensure that your heart is functioning normally. This could be a multiple-gated acquisition (MUGA) test or echocardiography.
Throughout treatment, you will continue to undergo routine cardiac function exams.
6. Lung Damage
The lungs may become inflamed or scarred as a result of trastuzumab deruxtecan. This condition is often referred to as pneumonitis or interstitial lung disease (ILD).
If you have any of the following, you should seek treatment right away.
- breathing difficulty
- a cough, whether or not associated with a fever
- any deteriorating or new respiratory issues
You might need a chest x-ray or CT scan to determine if you have ILD. You might need to temporarily halt treatment if Trastuzumab Deruxtecan scarred your lungs before receiving steroids to treat ILD. It can cause irreversible lung damage, which could deteriorate over time. Treatment can lessen symptoms, enhance the quality of life, and stop the condition from worsening.
7. Liver Changes: Trastuzumab Deruxtecan may affect the functioning of the liver. You need to get blood tests done to examine your liver function. Sometimes the doctors may suggest reducing the dosage or delaying the treatment if they discover any problems in your liver.
8. Nausea & Vomiting: As a result of the drug, one may experience sickness as in the symptoms of Nausea and vomiting, although one may not be actually sick. The symptoms can be treated by taking the right medications as prescribed by your doctor.
9. Diarrhea or constipation: You may go through diarrhea or constipation. However, not everybody experiences the same side effects. You must consult your doctor if diarrhea remains for more than 24 hours.
10. Cancer-related fatigue (extreme tiredness): One can experience fatigue and feel extremely drowsy. It is such that it won’t go away with rest or adequate sleep. Cancer related fatigue is the most common side effect anybody can experience after breast cancer treatment.
11. Sore mouth: Taking care of oral health is crucial during treatment.
12. Hair thinning or hair loss: Hair loss may be brought on by Trastuzumab deruxtecan. Any hair loss should only be momentary; once your treatment is finished, your hair will usually start to grow back.
13. Loss of appetite: You might not feel like eating. Maintaining your food intake can be achieved by eating small, regular meals or snacks. Ask your doctor or specialist about consulting a nutritionist if you have trouble maintaining a healthy weight.
14. Low potassium levels in the blood: To monitor your potassium levels before and throughout therapy, you will have routine blood tests. If you have low potassium levels, your medical staff may suggest supplements. Your potassium levels should return to normal as soon as your therapy is through.
15. Other common side effects: Few other side effects of Trastuzumab deruxtecan are suffering Headaches, Skin rash, Dry eyes, Dizziness, Abdominal pain, and Indigestion. All these side effects are easily treatable.
References: