Overview
The demand for healthcare technology is surging, but so are the stakes. Every line of code written for a clinical application has the potential to affect patient safety, compliance status, or financial stability. Hospitals, payers, and digital health innovators are under pressure to deliver software that integrates seamlessly with existing systems, scales with demand, and satisfies regulators like the FDA.
This is where a structured, end-to-end development process becomes critical. From discovery workshops to submission readiness, each stage lays the foundation for safe, effective, and compliant healthcare solutions. Skipping steps may save time in the short run, but it often results in costly rework, delayed launches, and heightened regulatory risk. In the following sections, we’ll walk through how to approach custom healthcare software development with discipline, you through how to approach custom healthcare software development with discipline, ensuring that what you build is ready for the clinic and for regulatory approval ensuring that what you build is ready for the clinic and for the regulator.
Why a Rigorous Process Matters
Healthcare software is different from typical enterprise applications. Beyond performance and usability, it must meet strict safety, quality, and compliance benchmarks. Regulators like the FDA require documented design controls, validation evidence, and traceability of requirements from concept to release. Industry standards such as IEC 62304 and ISO 14971 outline lifecycles and risk management processes to ensure software is built and tested against clear criteria.
A rigorous process provides three advantages. First, it reduces regulatory uncertainty by aligning with established standards from the beginning rather than treating compliance as an afterthought. Second, it ensures usability by capturing clinical workflows early and validating them with real users. Third, it lowers long-term costs by minimizing late-stage rework, which is far more expensive once architecture and code are already in place.
Phase 1: Discovery & Requirements
Discovery is where missteps can save or sink a project. Too many healthcare builds fail because the team never sat down with the people who actually use the system. In this phase, structured workshops include physicians, nurses, administrators, compliance officers, and even patient advocates. Each group contributes pain points and must-haves.
For example, when mapping out an oncology scheduling system, requirements are not just “book appointments.” They include handling infusion chair availability, drug preparation windows, and cross-team scheduling with pharmacy. If these realities are ignored at the discovery stage, the result is a “working” system that creates new bottlenecks instead of solving old ones.
The outcome of this phase is a requirements specification that aligns with both clinical realities and regulatory needs such as HIPAA audit logs and secure role-based access.
Phase 2: Design Control & Risk Management
Once the requirements are locked, the design process begins under strict controls. This is where FDA expectations start to surface. Design inputs are mapped to design outputs, and every requirement is linked to a validation plan. A traceability matrix is built so that when regulators ask, “How did you ensure this requirement was tested?” the answer is immediate.
Risk management is also front and center. Using ISO 14971 frameworks, teams identify hazards like incorrect dosage calculations, missed alerts, or data integrity failures. Each risk is scored, and mitigation strategies are documented before a line of code is written.
Consider a telehealth module that transmits vital signs. Risk analysis would highlight what happens if readings are delayed or lost. The design response could be automated alerts, redundant storage, or patient-facing notifications. This level of planning ensures the software is resilient and defensible during FDA review.
Phase 3: Development & Validation
With requirements and design controls in place, development can begin. In healthcare, coding is never just about features; it is about meeting compliance and patient safety standards as the product is built. Teams use agile or iterative cycles but maintain rigorous documentation so each sprint produces both working code and evidence of compliance.
Validation is a continuous process. Unit tests confirm modules work as expected, integration tests verify data flows across systems like EHRs or medical devices, and user acceptance testing involves clinicians simulating real workflows. For example, in a medication ordering module, validation checks might include verifying that dosage ranges are enforced, audit trails are created for each order, and clinical alerts fire correctly.
The FDA’s “General Principles of Software Validation” emphasize this cycle of building and testing. By embedding validation into every sprint, you avoid the trap of coding first and scrambling for evidence later. The result is software that is both functional and defensible under regulatory scrutiny.
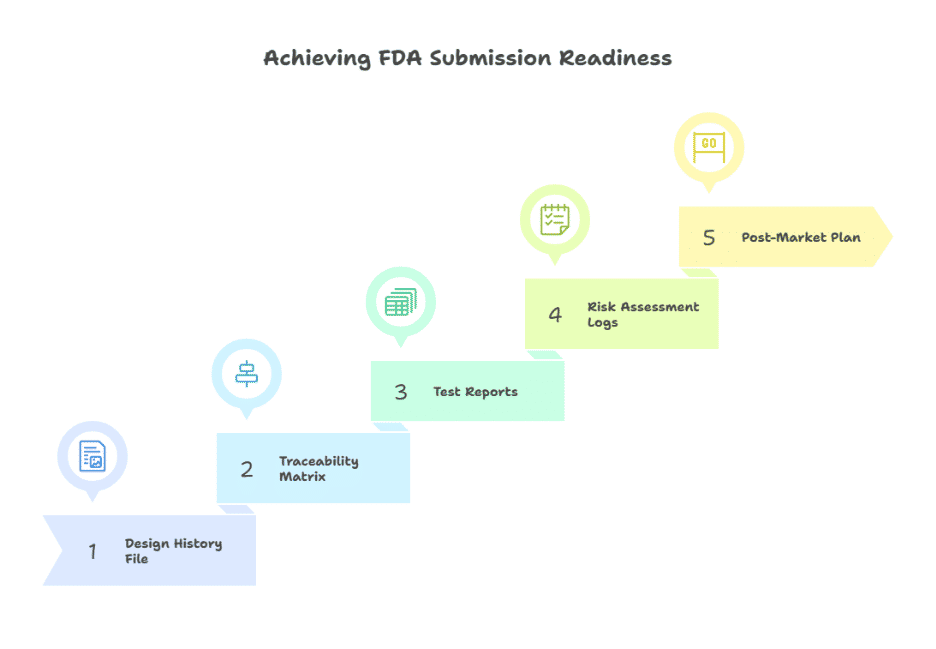
Phase 4: Submission Readiness & Post-Market Planning
When development is complete, the focus shifts to documentation and regulatory preparation. A Design History File (DHF) captures every requirement, risk assessment, test result, and design change. A traceability matrix shows exactly how each requirement was validated. This body of evidence reduces friction when submitting for FDA clearance or audit review.
Submission readiness is not just paperwork. It involves preparing the software for inspection: source code repositories are organized, validation scripts are reproducible, and quality assurance reports are accessible. Organizations that treat compliance as an ongoing practice, rather than a one-time exercise, are best positioned at this stage.
Post-market planning is equally important. Once the software is in production, ongoing monitoring for risks, periodic security updates, and change management processes are expected. For example, an app that captures remote patient monitoring data should have a framework for logging anomalies, reporting incidents, and rolling out validated updates without disrupting care delivery.
Mini Case Snapshot
When an Oklahoma-based behavioral health provider needed MU3 certification for its existing EMR, Nalashaa stepped in to lead the transformation. The project required regulatory gaps to be closed swiftly, even while another team continued ongoing enhancements.
Nalashaa’s analysts began with a compliance audit, identifying areas where the system fell short of Meaningful Use Stage 3 standards. Clinician workflows were mapped and realigned with certification requirements. Engineers then conducted mock certification sessions using test scripts and prepared the platform for its evaluation by the Drummond Group. These efforts enabled the system to earn MU3 certification without disrupting sales or compromising user experience.
Conclusion & Backlink CTA
Custom healthcare software is not just code; it is a regulated product that lives at the intersection of patient safety, compliance, and innovation. An end-to-end approach that begins with discovery and continues through submission readiness is the only way to deliver solutions that meet both clinical expectations and FDA scrutiny.
Organizations planning new digital health initiatives should choose a partner experienced in guiding projects across this full lifecycle. Working with a trusted custom healthcare software development company ensures the process is structured, compliant, and aligned with your long-term goals.
OG: